In a recent development, AstraZeneca, the pharmaceutical company behind widely administered COVID-19 vaccines such as Covishield and Vaxzevria, has acknowledged that their vaccines may lead to a rare but serious side effect known as Thrombosis Thrombocytopenia Syndrome (TTS). Here are the key points:
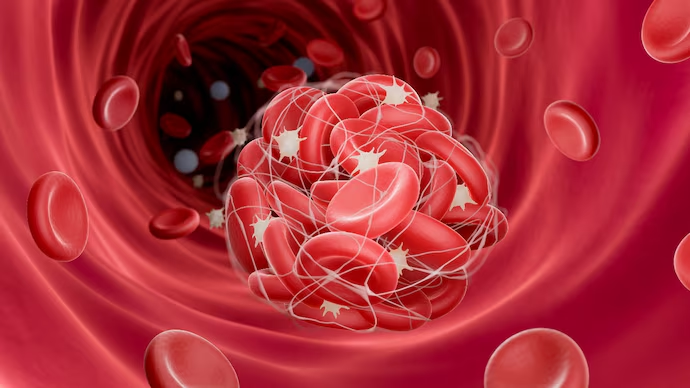
- What is TTS?
- TTS refers to the formation of blood clots in blood vessels, often accompanied by a low platelet count.
- It occurs in very rare cases following the use of certain types of vaccines.
- AstraZeneca’s Admission:
- Vaccine Technology:
- Covishield, produced by the Serum Institute of India, uses a viral vector platform.
- The vaccine contains a modified chimpanzee adenovirus (ChAdOx1) that carries the COVID-19 spike protein into human cells.
- This technology has also been used for vaccines against other viruses, such as Ebola.
- World Health Organization (WHO) Statement:
- In 2023, the WHO recognized TTS as a new adverse event following immunization with COVID-19 non-replicant adenovirus vector-based vaccines.
- This specifically includes the AstraZeneca ChAdOx-1 vaccine and the Johnson & Johnson Janssen Ad26.COV2-S vaccine1.
- Awareness and Management:
- TTS is a serious and life-threatening adverse event.
- The WHO issued interim emergency guidance to increase awareness about TTS in the context of COVID-19 vaccination and assist healthcare providers in assessing and managing potential TTS cases1.
Remember that while COVID vaccines have played a crucial role in preventing deaths, reports of these extremely rare but potentially serious immune-mediated events have also been documented. As always, it’s essential to stay informed and consult with healthcare professionals regarding vaccination decisions1. 🌟
Leave a Reply